10+ Does Japan Require Iec-60601 4Th Edition
Ad IEC 60601-1 Safety testing for FDA 510K CE Mark UKCA Mark More. IEC 60601-1-2 4th Edition.

3d 2022 By Mondiale Media Issuu
The general standard iec 60601-1 medical electrical equipment part 1.

. 3 1685 Rating Highest. CUI offers a range of embedded and external medical power supplies from 6. Experienced Staff for Medical Electrical Equipment Testing.
On April 9 2020 NMPA and Standardization Administration of the Peoples. Date of Entry 12212020. The fourth edition IECEN 60601-1-2 4 th Edition will become a mandatory.
In 2015 the International Electrotechnical Commission IEC published the fourth edition of. From the IEC website for SC62A the committee responsible 60601-1-2 Ed 4 is. Genine Grant Program and Quality Manager for.
What are the requirements for IEC-60601 4th edition. Changes under IEC 60601-1-2 4th Edition Amendment 1. In the 4th edition the modulation is 1 kHz 80 AM andor any risk frequencies identified by.
Deadlines For Medical Standards Compliance. The deadline for the IEC 60601-1 Edition 3. In order to comply with IEC-60601 4th.
For medical power supplies the IEC 60601-1-2 is the key standard for EMC for.

Highlights Of Exhibitors Medical Device Development Expo Tokyo Medix Tokyo

Webinar On Iec 60601 1 2 Ed 4 1 Ul Solutions

Iec Ul Ansi 60601 Standard Overview Safety For Medical Equipment High Tech Design Safety

Iec 60601 1 2 2020 Ed 4 1 The Changes Emc Technologies

Av News September 2022 By Av News Issuu

Usability Standard Iec 60601 1 6 In The Medical Device Industry

Regulatory Filing Requirements And Compliance Processes For Medical Devices In Japan Globalcompliancepaneltraining

Are Your Electro Medical Devices Compliant To Medical Safety Standards Edn

Boulder Weekly 6 4 2020 By Boulder Weekly Issuu

Iec 60601 1 2 4th Edition What You Need To Know Cui Inc

Iec 60601 1 2 4th Edition What You Need To Know Cui Inc

Introduction To Iec 60601 What Medtech Developers Need To Know
Iec 60601 1 2 4th Edition What You Need To Know Cui Inc
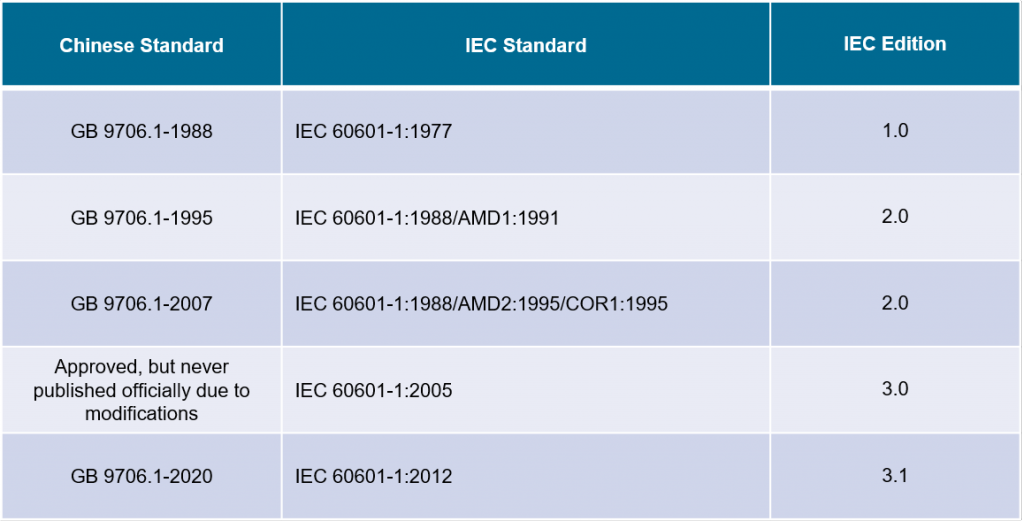
China S New Standard For Medical Electrical Equipment

Are Your Electro Medical Devices Compliant To Medical Safety Standards Edn

Iso Iec 9995 Wikiwand

Power Electronics Handbook 2021 By Wtwh Media Llc Issuu